https://www.nature.com/articles/s41467-025-63929-7
http://english.cas.cn/newsroom/research_news/life/202510/t20251014_1089412.shtml
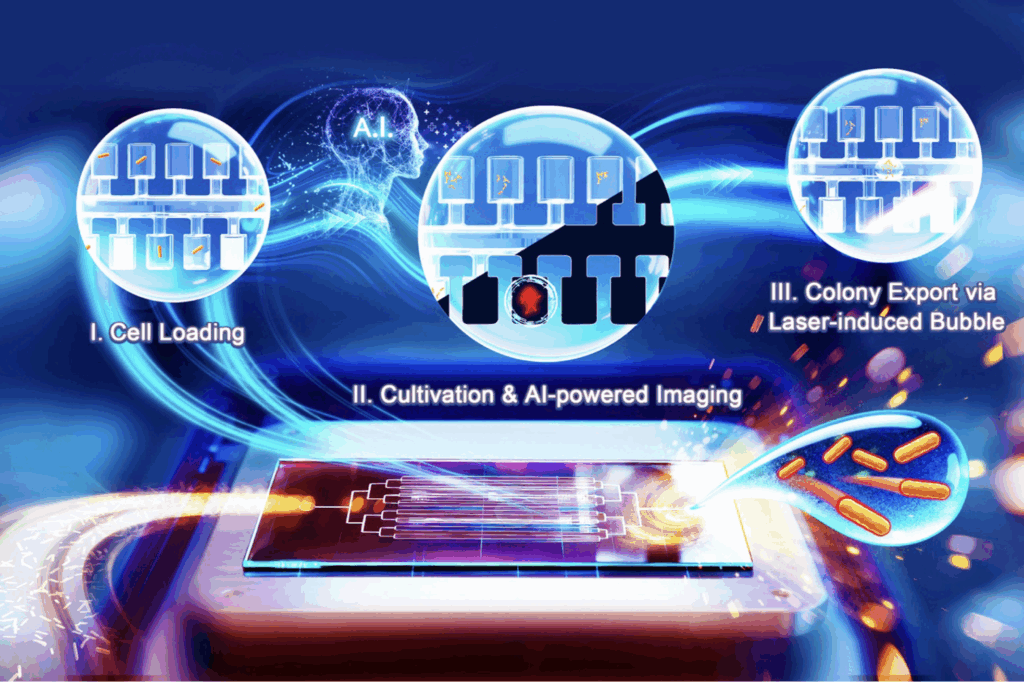
The group around Jian XU from the CAS Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) has developed a fully automated “Digital Colony Picker” (DCP). This device identifies and retrieves high-performance microbial clones by simultaneously monitoring their growth and metabolite production—eliminating the need for culture plates, sampling needles, or manual picking.
Designed for the “design-build-test-learn” framework widely adopted in synthetic biology, the DCP streamlines the traditionally slow, labor-intensive “test” phase into a fast, parallel workflow with little hands-on time. It has a microfluidic chip containing 16,000 addressable microchambers that isolate single cells and track their expansion into micro-colonies. An integrated AI engine conducts time-lapse analysis of both brightfield and biosensor signals to quantify growth kinetics and metabolite production in real time. Once target colonies are identified, a laser-induced bubble technique exports them as droplets directly into standard culture plates. This contact-free transfer minimizes cross-contamination and preserves cell viability.
The equipment which was tested for identifying high-yield or lactate-tolerant Zymomonas mobilis mutants is broadly applicable to adaptive evolution studies, functional gene discovery, and phenotype screening across diverse microbial species.
The iMAPS program which includes use of this device has been described in ore details under https://imaps.info/