https://www.sciencedirect.com/science/article/pii/S2950155525000667?via%3Dihub
https://www.cas.cn/syky/202511/t20251125_5089765.shtml
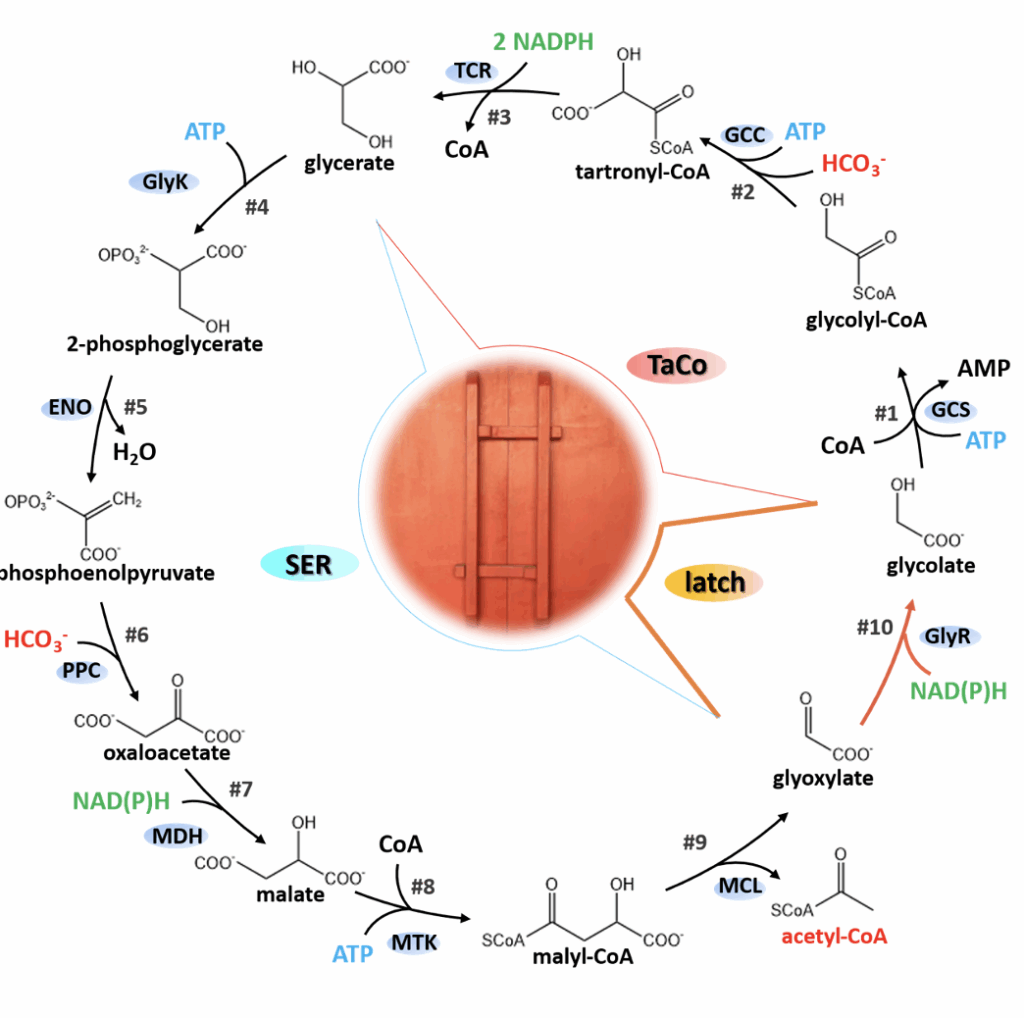
A research team at the CAS Tianjin Institute of Industrial Biotechnology has proposed a novel artificial carbon fixation pathway—LATCH which comprises 10 completely known enzymatic steps. Each cycle converts two molecules of HCO₃⁻ into one molecule of acetyl-CoA, requiring only adenosine triphosphate (ATP) and reduced coenzyme II for energy. Kinetic and thermodynamic modeling analysis shows that it is a linear autocatalytic cycle structure without kinetic traps or thermodynamic barriers, possessing high feasibility and potential for continued development. It can provide insights for improving the efficiency of systems such as photosynthetic microorganisms, plants, and engineered cell factories.
Regarding the selection of parental modules, the research team referenced research on the serine cycle and designed a modified version of the serine cycle, simplifying the pathway structure and bypassing the inefficient steps involving hydroxypyruvate, thus enabling the pathway to function effectively in the heterologous host *E. coli*. Simultaneously, the team replaced the amino acid deamination and transamination steps in the serine cycle with a decarboxylation process, forming an MCG cycle free from formic acid dependence. This cycle can further convert glycerate 3-phosphate produced by processes such as the Calvin cycle and glycolysis into acetyl-CoA in a negative carbon mode. The study also referenced a series of photorespiration bypass concepts developed for recovering the Rubisco byproduct glycolate-2-phosphate, among which the TaCo module, due to its artificial carboxylation reaction, theoretically has a maximum yield of 150%. This study found that by introducing glyoxylate reductase as a key step to act as a “molecular latch,” the natural serine cycle and the artificially carboxylated module TaCo can be recombined, resulting in a functional transformation—from two parent modules dependent on organic substrates to a complete carbon-fixing cycle.
Based on the LATCH cycle formed by module integration, kinetic analysis shows that this pathway is a linear autocatalytic cycle, theoretically avoiding kinetic traps while eliminating the need to establish complex regulatory relationships. Meanwhile, eight steps in the pathway receive thermodynamic support from adenosine triphosphate (ATP), reducing power, or high-energy substrates, and the remaining two lyase-catalyzed processes do not pose thermodynamic bottlenecks. These inherent advantages at the stoichiometric, kinetic, and thermodynamic levels lay the foundation for the continued development and application of LATCH.