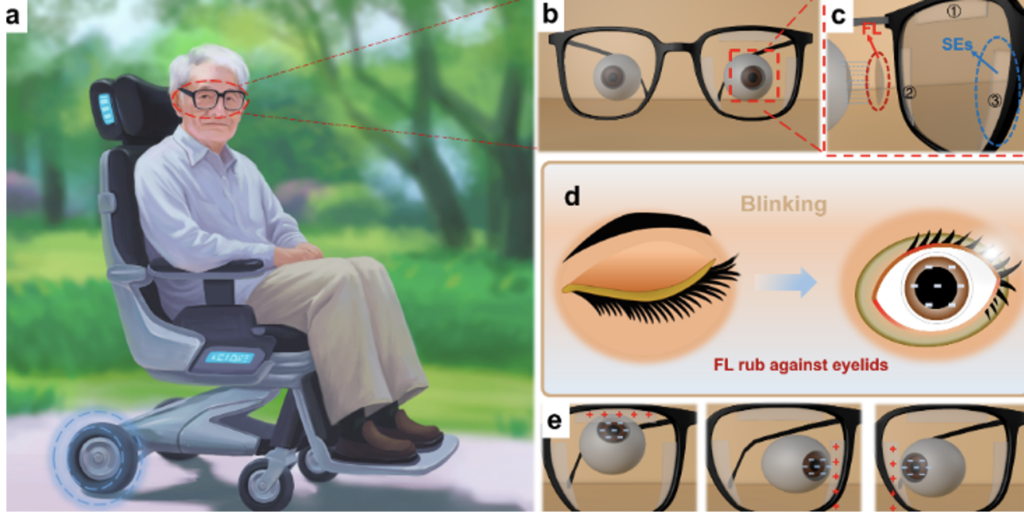
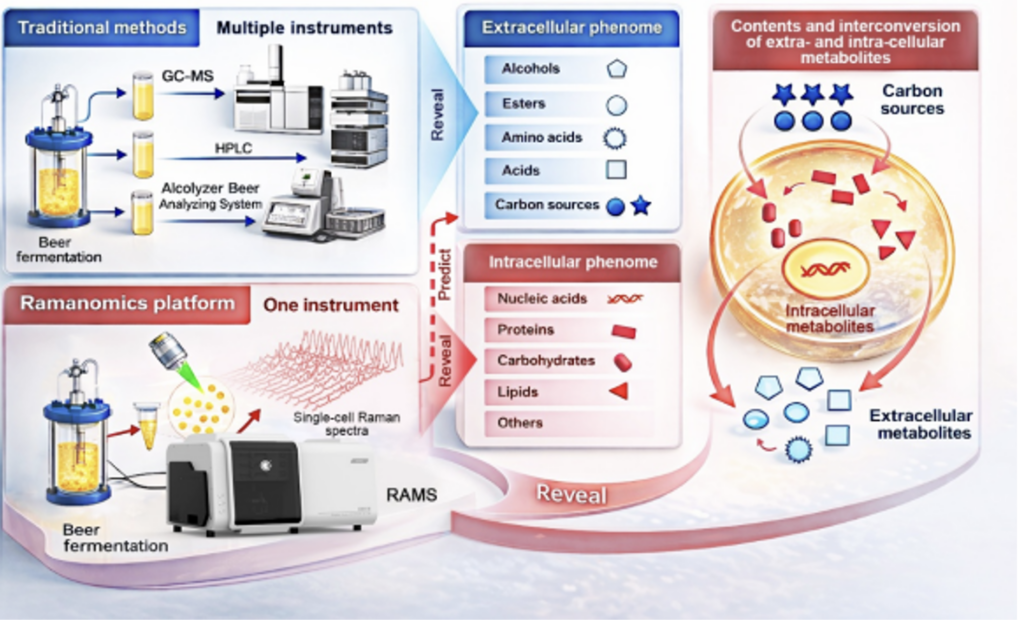
https://spc.jst.go.jp/news/250903/topic_2_03.html
A “China Blue Carbon 2025” Blue Book was released in Qingdao. The Blue Book project was led by the Marine Carbon Neutrality Center of the Ocean University of China, and had invited more than 70 experts and scholars from over 30 institutions in China and abroad to conduct joint special research.
The blue paper predicts that carbon dioxide absorption by China’s blue carbon ecosystems has been on the rise for over the past decade, reaching 500 million tons of carbon dioxide equivalent by 2035, at which point China will play a central role in global blue carbon contributions. By 2025, China’s total mangrove area will be approximately 303 square kilometers, with a total carbon storage of 6.03 million tons; seagrass beds will be approximately 265 square kilometers, with a total carbon storage of 2.3 million tons; and coastal salt marshes will be approximately 2,980 square kilometers, with a total carbon storage of 91.55 million tons.
The paper also notes that carbon absorption by shellfish and algae farming in China’s coastal waters has increased over the past 20 years. At the same time, China’s marine energy has also developed, with its offshore wind power capacity now number one in the world and its marine primary and secondary industries achieving “carbon minus” status.
According to the president of Ocean University of China, the university aims to achieve synergistic effects on the ecosystem, society, and economy by developing seagrass bed restoration technology, to building a blue carbon resource survey and calculation system, and even developing technologies to track and treat the sources of coastal pollutants.