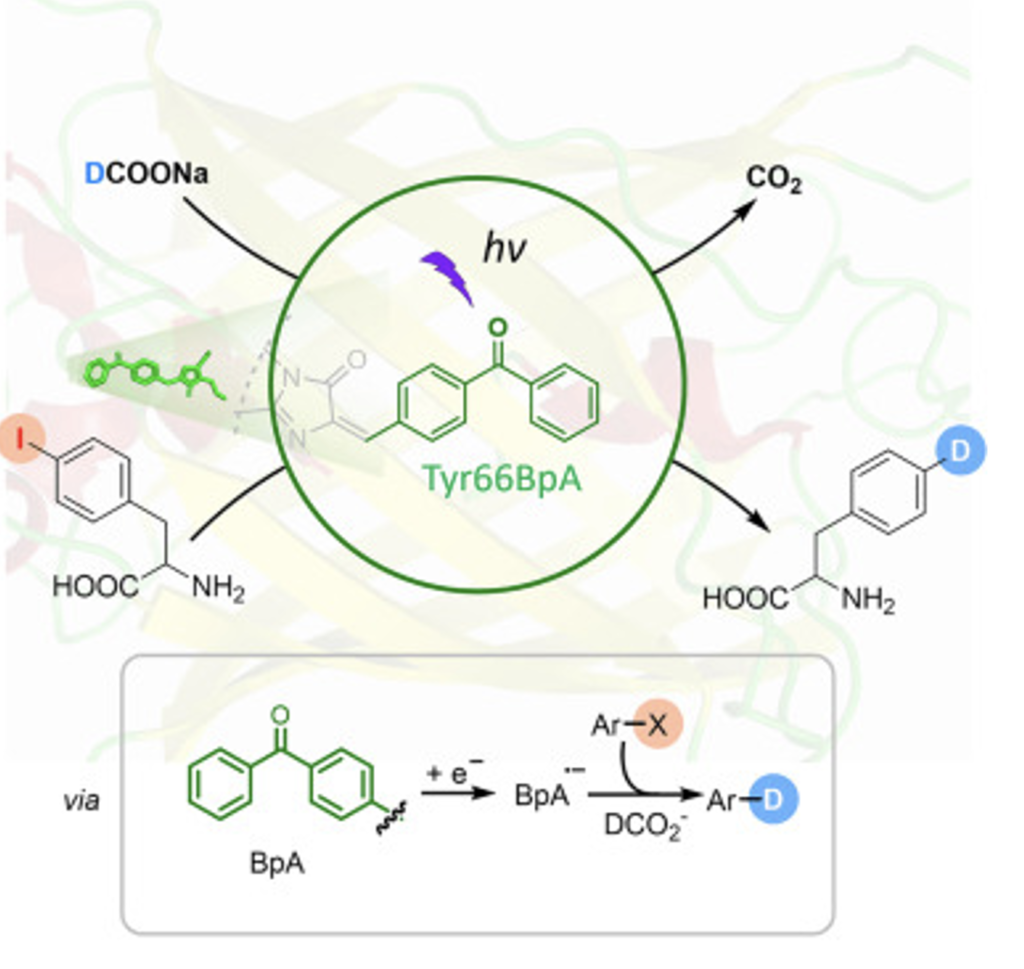
A benzophenone photocatalyst encoded in a yellow fluorescent protein, reductive photodehalogenase (RPDase), can proficiently mediate the biocatalytic hydrodehalogenation and deuterodehalogenation of aryl halides. Unlike natural metal-cofactor-dependent dehalogenases evolved for the bioremediation of specific substrates, this metal-free photoenzyme operates in combination with formate via an entirely unnatural catalytic mechanism and exhibits marked substrate generality. Taking advantage of the biorthogonality of RPDase and the genetic code expansion method, a whole-cell photobiocatalyst using recombinant Escherichia coli cells that express RPDase was developed.