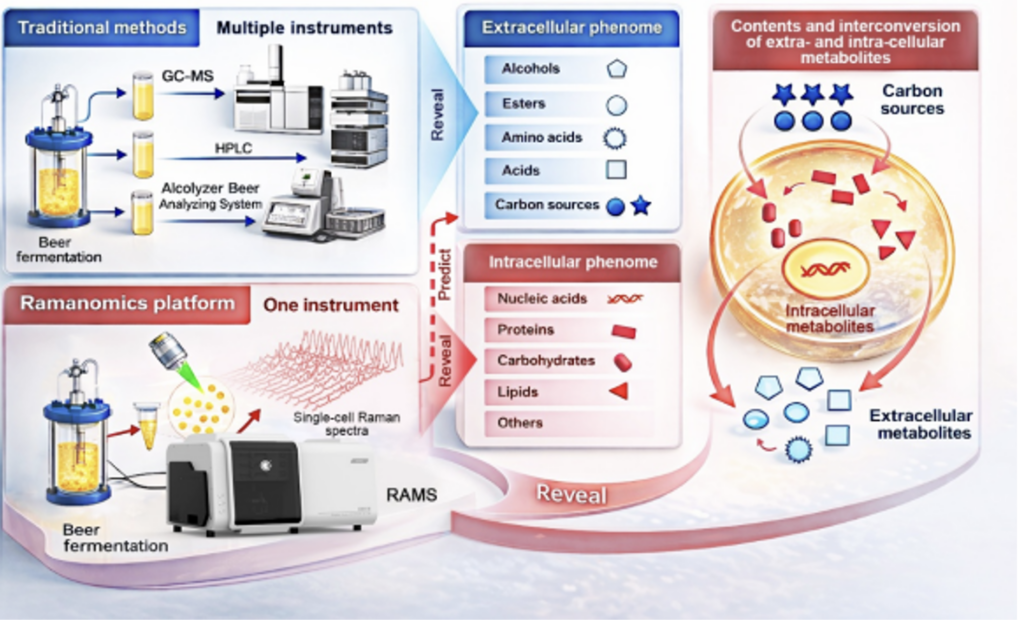
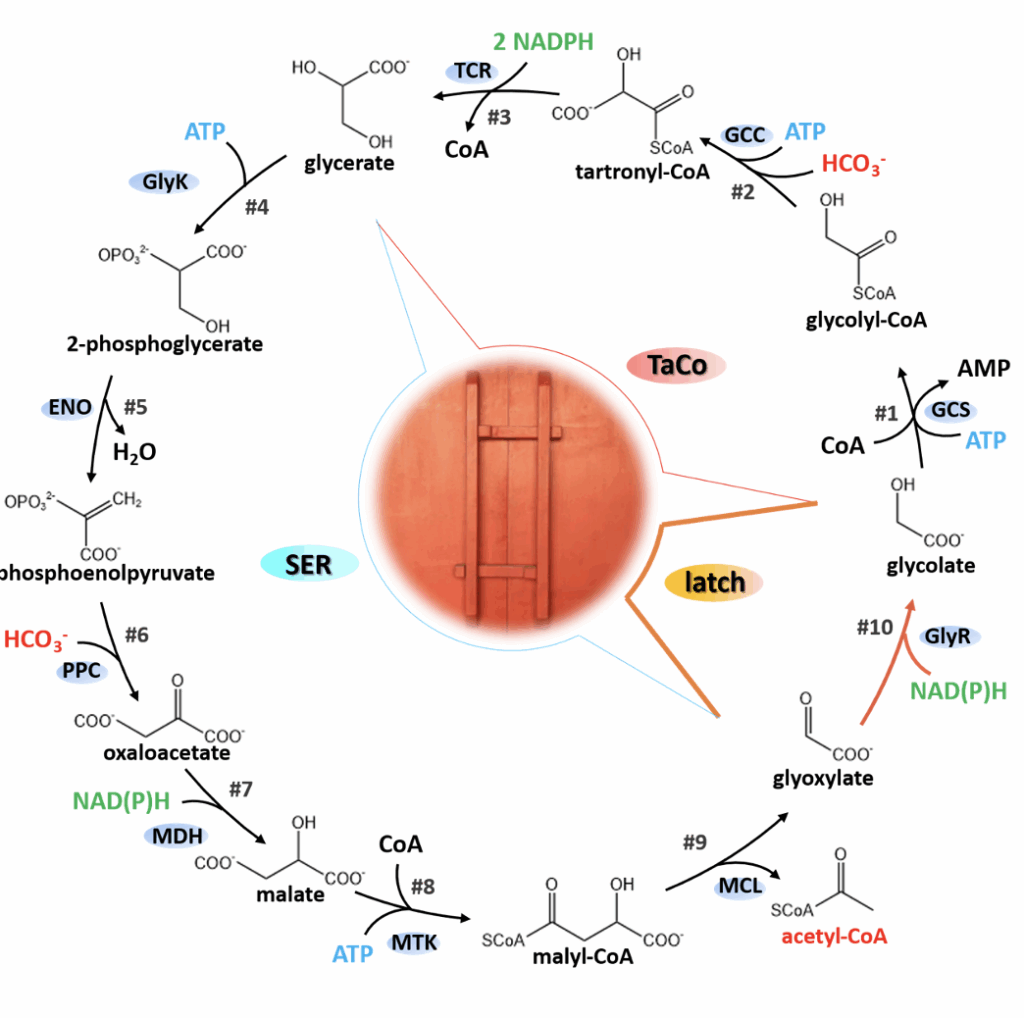
http://qibebt.cas.cn/news/zyxw/202409/t20240922_7378447.html
From September 19th to 22nd, the 17th International Clostridium Conference was held in Qingdao, hosted by the International Clostridium Conference Organizing Committee, supported by the Bioenergy Research Laboratory of Qingdao Institute of Energy, the Key Laboratory of Solar Photovoltaic Conversion and Utilization, Shandong Energy Research Institute, and Qingdao New Energy Shandong Provincial Laboratory, and co-organized by the One Carbon Biotechnology Research Center and Green Carbon Editorial Department.
Since 1990, the International Clostridium Conference has been held every two years, and this Clostridium Conference is the second time to be held in China. On the afternoon of the 19th, the executive chairman of the conference, Researcher Li Fuli, director of the One Carbon Biotechnology Research Center, announced the opening of the conference. Director Lv Xuefeng delivered a speech on behalf of the Qingdao Institute of Energy and introduced the construction and development of the institute to the delegates.
This conference invited about 150 scholars and guests from domestic and foreign academic and business circles to attend the conference, including more than 50 foreign experts from Germany, the United States, France, South Korea, the United Kingdom, Italy and other countries. The conference was divided into four parts according to the topic direction: physiology and systems biology, genetics and synthetic biology, metabolic engineering and raw material utilization, industry and new applications. More than 40 oral speakers shared the latest research results with the participants, discussed future research directions, and exchanged problems and challenges encountered in Clostridium research and industrialization engineering.
As a valuable platform for scientific exchange and cooperation, this conference will further promote the development of Clostridium research. At the same time, the successful holding of this conference is of great significance to enhancing the influence of the institute in the field of Clostridium research. (Text/Photo by Ma Xiaoqing)