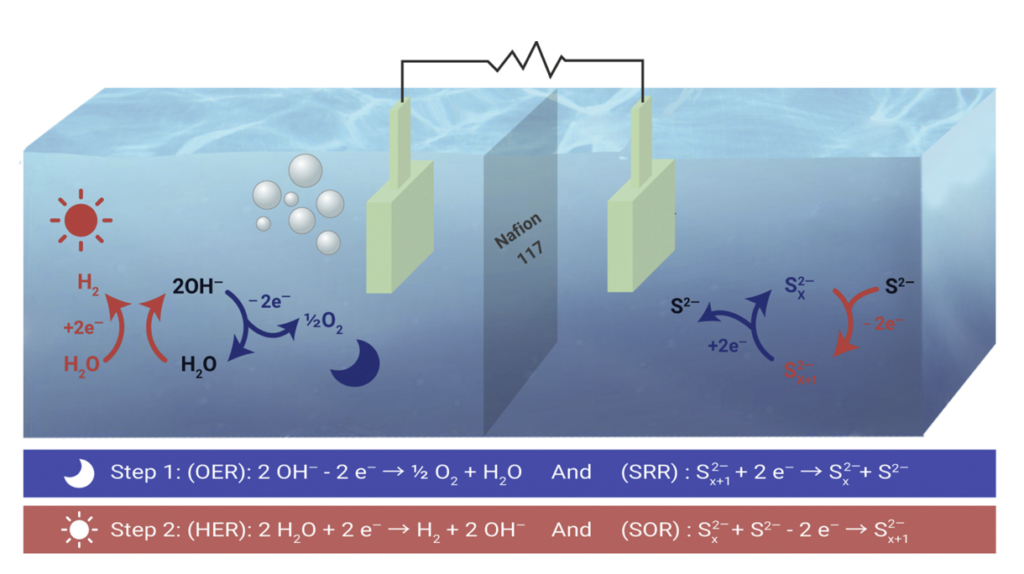
DENG Denghui’s group at the Dalian Institute of Chemical Physics of the Chinese Academy of Sciences wants to use hydrogen as a green energy carrier to balance peaks and troughs in power consumption. To this end, they use polysulfides as a mediator in a galvanostat, lowering the reduction potential for the formation of hydrogen to + 0.82 V at 100 mA/cm2, 60% below the value for the direct electrolysis of water. The mediator is then reduced again during nighttime operation at -1.81 V. The electrode is made of a CoNi nanoalloy encapsulated in N-doped graphene (CoNi@NGs), protecting it from sulfide corrosion. Using this “chainmail catalyst,” the researchers produced hydrogen in high space-time yields for 500 hours without poisoning the electrode.
DOI:https://doi.org/10.1016/j.xinn.2021.100144