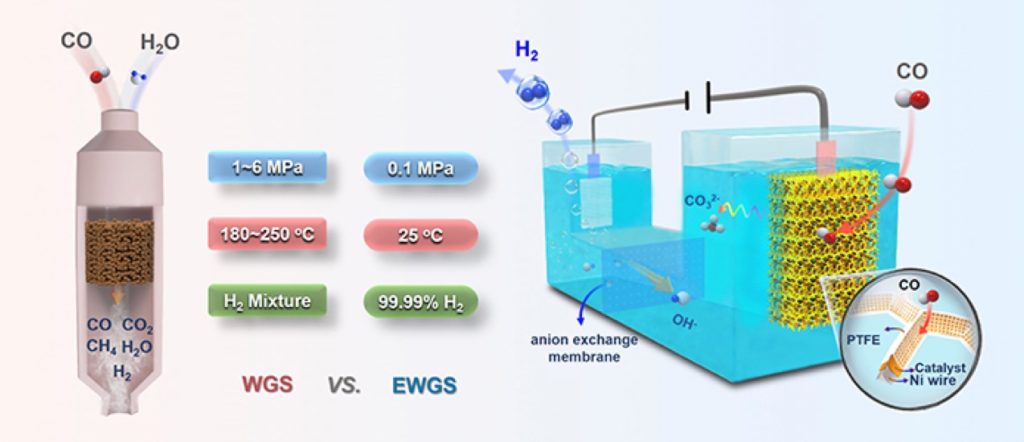
The team around DENG Dehui has found a new way for a highly energy-efficient conversion of water to hydrogen at room temperature by using an electrochemical vapor shift reaction. Water vapor shift reaction (CO + H2O → H2 + CO2) is the main method for industrially producing hydrogen on a large scale. However, the process is carried out at high temperature (180 ° C – 250 ° C) and high pressure (1.0 – 6.0 MPa). DENG’s team opted for an electrochemical method: CO is oxidized at a Pt/Cu anode, and the generated CO2 reacts with KOH to form potassium carbonate; at the cathode, water is directly reduced to high-purity hydrogen with nearly 100 % Faraday efficiency. Current density reached 70mA/cm2 at 0.6V, and the catalyst was highly active for 475 hours.
CAS news release, January 15, 2019
© CAS Dalian Institute of Chemical Physics

