https://www.cas.cn/syky/202506/t20250616_5073220.shtml
Research on the anti-aging human mesenchymal progenitor cell technology system has made progress
With the increase of age, the depletion of stem cell reserves and the resulting decline in tissue regeneration and homeostasis maintenance ability are key characteristics of body aging and aging-related diseases. However, whether stem cell depletion is a cause or effect in the aging process, and whether exogenous stem cell transplantation can effectively delay aging, are unsolved scientific problems.
Research Groups of the CAS Institute of Zoology and Xuanwu Hospital, Capital Medical University, constructed an engineered human anti-aging mesenchymal progenitor cell (SRC) with anti-aging, anti-stress and anti-malignant transformation capabilities, and verified its effect of delaying multi-organ aging in a primate, providing a new cell therapy paradigm for human aging intervention.
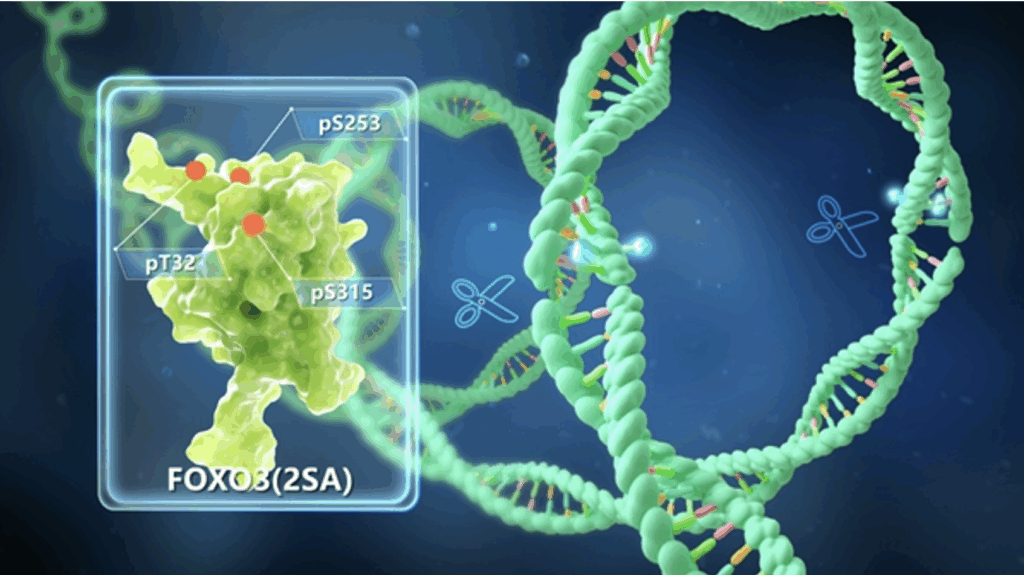
Back in 2011, the research team first achieved precise targeted correction of premature aging pathogenic genes in human pluripotent stem cells, proving that genetic modification of gene circuits can reverse the aging clock of human cells, laying a theoretical foundation for the generation of engineered longevity cells. After more than ten years of exploration, the team has constructed an anti-aging human mesenchymal progenitor cell (SRC) technology system – SRC 1.0 and SRC 2.0 through synthetic biology reprogramming. SRC 1.0 (2017) precisely edited the oxidative hub gene NRF2, strengthened the cell’s endogenous antioxidant defense network, and established enhanced cells with high metabolic activity and high genomic homeostasis. SRC 2.0 (2019) performed dual-site engineering modification of the longevity node gene FOXO3, reconstructed the spatiotemporal regulation network of phosphorylation signals, and endowed engineered progenitor cells with transplantable enhanced functional characteristics.
This study systematically analyzed the anti-aging phenotype of SRC 2.0. The results showed that FOXO 3-enhanced SRC exhibited significant anti-aging activity, strong environmental adaptability and excellent safety characteristics, and could effectively resist the aging microenvironment and avoid tumorigenic risks.
The team then selected elderly crab-eating macaques with a physiological state equivalent to that of healthy humans aged 60 to 70 as experimental models to conduct a 44-week SRC intervention study. Multiple intravenous injections of SRC did not lead to adverse events, and histopathological evaluation also ruled out the damage and tumorigenic risks of transplanted cells, confirming the safety and immune tolerance of SRC transplantation in non-human primate models.
In summary, through the systems biology research paradigm, this study comprehensively evaluated the intervention effect of SRC on primate aging models. The results showed that SRC transplantation can significantly delay the aging process of multiple organs in monkeys and reconstruct the homeostasis of the body, which is manifested in improving cognitive function, improving multi-tissue degenerative lesions, reducing the accumulation of senescent cells, and enhancing genome stability. At the gene expression level, SRC transplantation can achieve systematic rejuvenation of the aging-related gene expression network of more than half of the tissues, and reverse the expression profile of aging-related genes in key systems in the single-cell dimension. Aging clock analysis based on machine learning confirmed that the biological age of immature neurons was reversed by 6 to 7 years, and the biological age of oocytes was reversed by 5 years.
At the same time, mechanism exploration showed that exosomes released by SRC played a core role in promoting cell rejuvenation, inhibiting chronic inflammation, and maintaining genome and epigenome stability.