https://www.cas.cn/cm/202311/t20231114_4985649.shtml
https://www.nature.com/articles/s44160-023-00424-1
While preparing for a future Mars mission, researchers from CAS University of Science of China (USTC) and Technology have developed the following rationale:
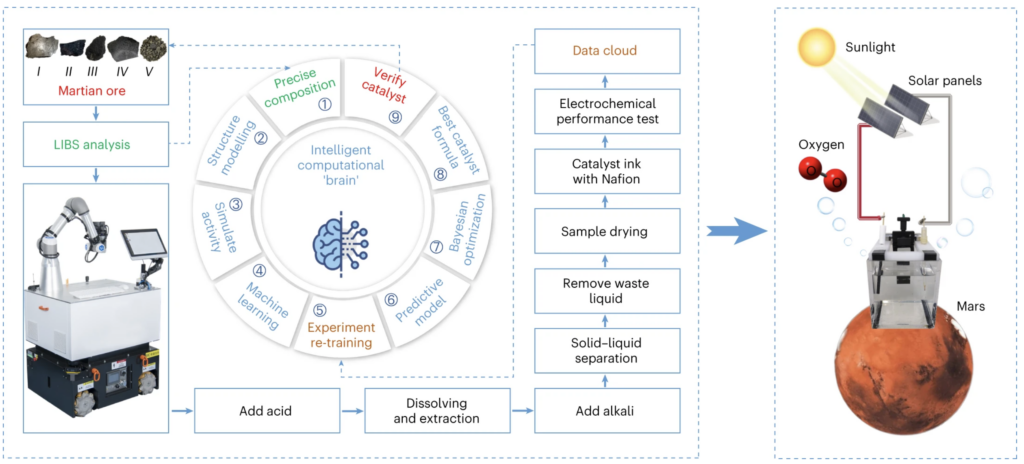
The oxygen content in the Martian atmosphere is extremely low. However, recently it was found that there is a large amount of water on Mars. Thus, the use of solar power on Mars could provide electricity to parse out oxygen from the water. This will need a suitable catalyst, but the cost of its transportation from Earth to Mars is forbidding. Therefore, the catalyst should be synthesised locally on Mars. However, low temperature, low pressure and high radiation on Mars is unfavorable for R&D on Mars after human landing. Thus, a “machine chemist” should be developed to achieve this task.
Using Martian meteorite materials and a machine-learning model derived from both first-principles data and experimental measurements, the USTC researchers have developed an automated synthesis and intelligent optimization of catalysts for the required oxygen evolution reaction. The entire process, including Martian ore pretreatment, catalyst synthesis, characterization, testing and the search for the optimal catalyst formula was performed without human intervention.
To arrive at this result, the system studied more than 50,000 related chemical papers, used its ‘intelligent brain’ to think and design a basic formula, and then did experiments and constantly adjusted the ratio according to the results. After six weeks it had found the best formula.
The resulting polymetallic material (comprising Mn, Fe, Ni, Mg, Al and Ca) catalysed the oxygen evolution with an overpotential of 445.1 mV at a current density of 10 mA cm−2, maintained for 550,000 s (153 hrs). Further, the stress test at −37 °C, which mimics the temperature condition on Mars, confirmed that it can steadily produce oxygen without apparent deterioration, suggesting that it can work in the harsh conditions on Mars.