https://www.cas.cn/syky/202507/t20250722_5077265.shtml
https://advanced.onlinelibrary.wiley.com/doi/full/10.1002/advs.202504669
In the natural environment, mammals reproduce through strict sexual reproduction, effectively ensuring the genetic diversity of the population. However, in many fields such as agricultural animal husbandry, endangered animal protection, pet cloning, disease model creation and regenerative medicine research, people often need to obtain individuals with completely identical genomic genetic material. Somatic cell cloning technology is to transplant somatic cell nuclei into enucleated oocytes, reprogram them to form omnipotent embryos, and then develop into cloned animals with exactly the same genome as the donor cell nucleus. However, for a long time, somatic cell cloning technology has been severely restricted in its application in actual production and scientific research due to its low cloning efficiency. How to improve the efficiency of somatic cell cloning is the core issue in this field.
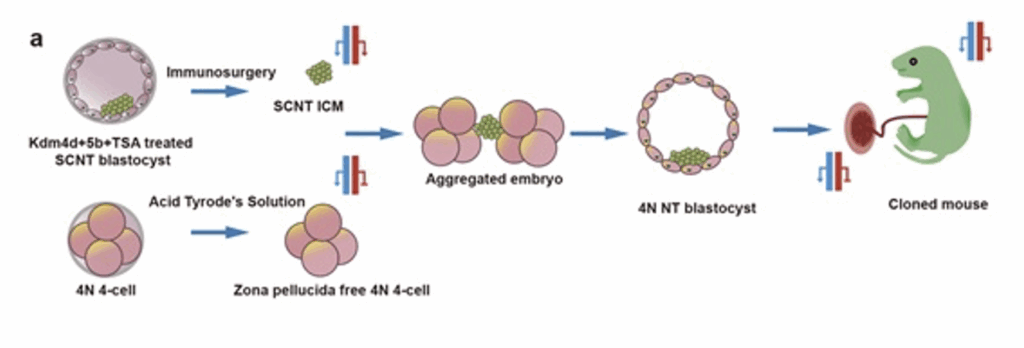
Somatic cell cloned embryos have complete oocyte cytoplasm and genomic DNA sequences consistent with donor cells. Their low development efficiency is mainly due to disordered gene expression caused by epigenetic abnormalities. Previous studies have found that there are multiple histone modification abnormalities such as H3K9me3, H3K4me3 and histone acetylation in pre-implantation somatic cell cloned embryos, and intervening in these abnormal histone modifications can effectively improve the pre-implantation development of embryos. Further, studies have found that atypical gene imprinting is missing in mouse somatic cell cloned embryos; and then by knocking out multiple genes in donor cells to simulate the imprinted expression state of major atypical imprinted genes, it was proven that repairing atypical gene imprinting can effectively improve the post-implantation development of somatic cell cloned embryos.
A research team from the CAS has treated somatic cell cloned embryos with the histone deacetylase inhibitor trichostatin A (TSA), and simultaneously injected mRNA encoding H3K9me3 demethylase Kdm4d and H3K4me3 demethylase Kdm5b into the embryos to intervene in abnormal histone modifications, effectively resolving the epigenetic barriers to pre-implantation development of somatic cell cloned embryos. At the same time, the tetraploid compensation technology was used to replace the trophoblast cells of cloned embryos, effectively addressing the problem of atypical gene imprint loss in the placental tissue of cloned embryos, thereby resolving the epigenetic barriers to post-implantation development of somatic cell cloned embryos. Through this combined somatic cell cloned embryo epigenetic barrier intervention technology strategy, a birth rate of about 30% of somatic cell cloned mouse transplanted embryos was achieved.
This study is expected to provide new technical strategies for cost-effective and efficient reproduction of large mammals through somatic cell cloning in the future.