https://www.cas.cn/syky/202506/t20250611_5072462.shtml
Proton exchange membrane water electrolysis is one of the key technologies for large-scale preparation of green hydrogen. The precious metal Pt has moderate hydrogen binding energy and excellent acid corrosion resistance. Platinum/carbon (Pt/C) is usually used as a cathode catalyst in commercial PEMs. However, under long-term operation or high reduction potential conditions, Pt nanoparticles in Pt/C catalysts tend to agglomerate or fall off the carrier, resulting in a reduction in the number of active sites and a decline in overall catalytic performance. In order to maintain an efficient and long-life water electrolysis process, it is often necessary to increase the Pt loading, which greatly increases the cost of the catalyst.
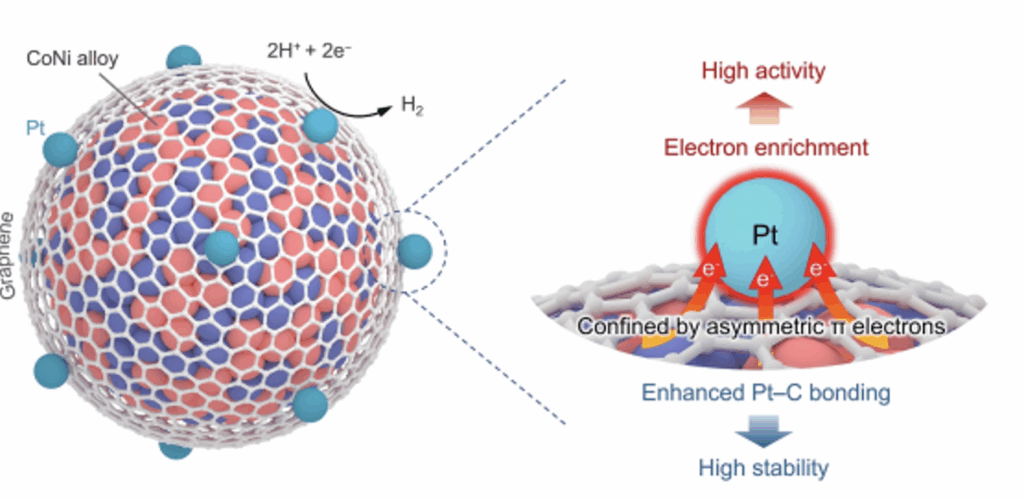
A team at CAS DICP found that in the structure of the non-precious metal cobalt-nickel (CoNi) nanoalloy armor catalyst encapsulated in a single-layer graphene, the electron transfer from metal to carbon and the electronic interaction between metal and graphene can form enriched asymmetric π electron states on the surface of the graphene armor. Through atomic layer deposition technology, the team achieved precise deposition of single-atom Pt at asymmetric π electron enriched sites on the surface of the graphene armor. Combining a variety of in-situ spectroscopy and theoretical calculation studies, it was found that this asymmetric π electron has a unique electron confinement effect on Pt atoms: on the one hand, the electron transfer from CoNi nanoalloy to Pt atoms through graphene armor makes Pt electron-rich, optimizes the hydrogen adsorption energy of Pt sites, promotes hydrogen desorption, and improves the electrocatalytic hydrogen evolution activity of Pt sites; on the other hand, the asymmetric π electrons enriched on the graphene surface form a strong interaction with the 5d orbital of Pt, which improves the structural stability of the Pt site.
The PEM electrolyzer assembled by the team using this new armor catalyst can achieve a current density of 4.0 A cm−2 at a cell voltage of 2.02 V (without iR compensation), and can operate stably at a current density of 2.0 A cm−2 for more than 1,000 hours. The Pt loading on the cathode side of the electrolyzer is only 1.2 μgPt cm−2. In further scale-up experiments, the team used the catalyst to assemble a 2.85 kW PEMWE electrolyzer that also had excellent catalytic activity and stability, and operated stably for more than 300 hours at an industrial current density of 1.5 A cm−2, showing excellent industrial application potential. This work provides new ideas for the development of high-performance, long-life, and low-cost acidic water electrolysis hydrogen production catalysts.