https://www.globaltimes.cn/page/202503/1330753.shtml
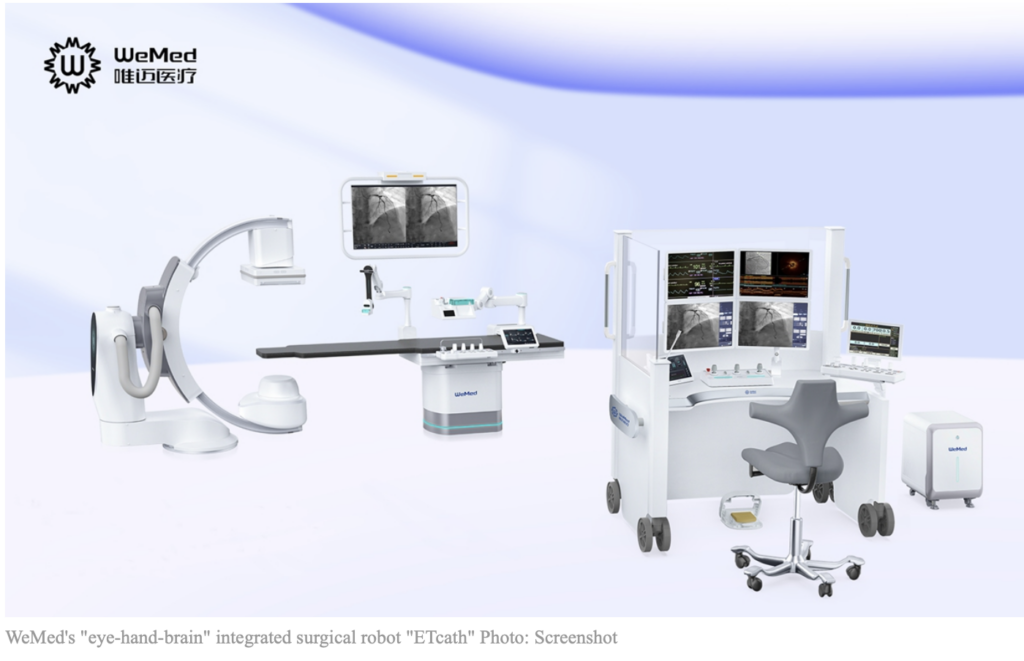
The National Medical Products Administration has approved the interventional surgical robot ETcath for market launch. ETcath was developed by the Beijing-based medical equipment firm WeMed and combines high-definition imaging, precise robotic arms, and an Al-empowered analysis database to enhance operational synergy during medical surgeries.
ETcath’s imaging system is capable to identify lesions with 0.1-millimeter-level precision, five times more accurate than the human eye. Its robotic arms can achieve submillimeter motion control with an error of less than 0.05mm and can sense minimal forces as low as 0.01 Newtons.
Besides, the Al-empowered analysis database stores millions of clinical case data, which can generate clinical case models within a second, and provides precise imaging data analysis, according to the post.