https://www.cas.cn/syky/202407/t20240731_5027917.shtml
https://www.nature.com/articles/s41467-024-50535-2
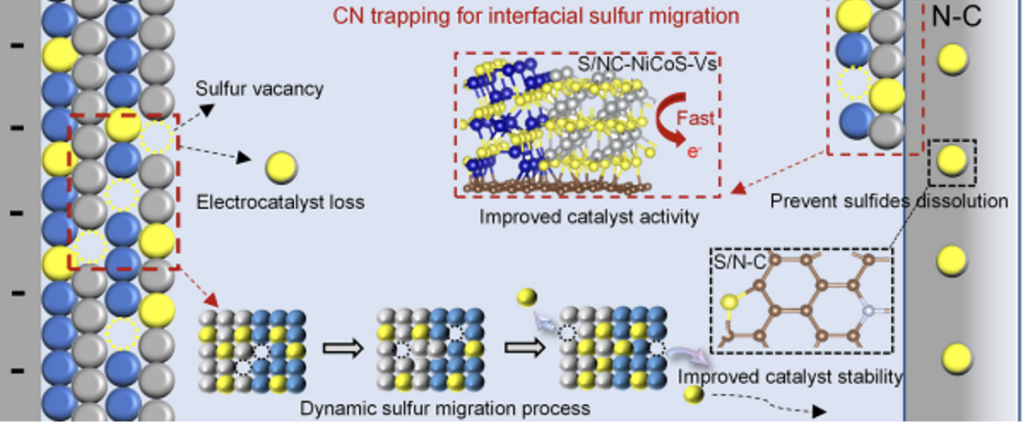
A team around Wang Erdong and Yang Bing at the CAS Dalian Institute of Chemical Physics have improved hydrogen evolution catalysts for electrolysis of seawater. They have constructed a nitrogen-doped carbon (CN) shell-encapsulated NiCoS heterostructure (CN@NiCoS) electrocatalyst to control interfacial sulfur migration and overcome the gap between activity and stability. In situ characterization and theoretical calculations reveal the process of sulfur migration and trapping in CN@NiCoS catalyst. The Ni3S2/Co9S8 heterointerface promotes the removal of sulfur atoms and forms sulfur vacancies. The migrating sulfur atoms are captured by the CN layer, inhibiting the irreversible dissolution of sulfur, thus improving the stability of the hydrogen evolution reaction. In addition, the sulfur-doped CN and sulfur vacancy (S-NC/Vs) sites formed during the dynamic migration and trapping process of sulfur atoms further regulate the d electronic structure of TMSs and enhance the hydrogen evolution reaction activity. Research has found that the overpotential at a current density of 10mA/cm2 in alkaline pure water and seawater media is only 4.6 and 8mV, and it can operate stably for 1,000 hours.