https://www.cas.cn/syky/202407/t20240719_5026474.shtml
https://www.sciencedirect.com/science/article/pii/S1385894724051799?via%3Dihub
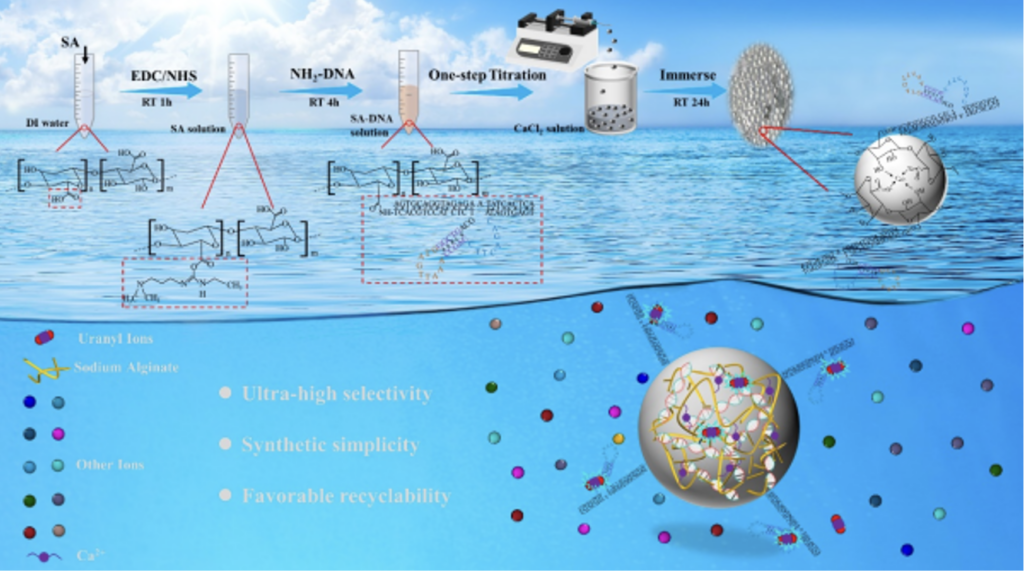
A team at CAS Qingdao Institute of Bioenergy and Process Technology has prepared a new type of sodium alginate-DNA material for the extraction of uranium from seawater. The microspheres can be used to selectively adsorb uranyl ions (UO22+). By using DNA as a selective adsorption site, the affinity of the microspheres is significantly improved, and selectivity is 43.6 times that of vanadium ions.
SA-DNA hydrogel microspheres are about 2 mm in size and contain a large number of micron-sized pores to enhance mass transfer and fully expose active sites. At 25 °C and pH=4.0, the maximum adsorption capacity of the microspheres is 189.5 mg⋅g-1. FTIR and XPS analysis showed that uranyl ions mainly coordinate with the P atoms and O atoms of the phosphodiester bonds in DNA, and chelate with the carboxyl groups on the microspheres.
At present, the land uranium reserves are limited, while there is abundant uranium in the ocean. Therefore, extraction from seawater can be used as another source of uranium to ensure that nuclear energy meets the needs of industrial development. Studies have found that adsorption is an effective method for extracting uranium from seawater, but due to the low concentration of uranium in seawater and the complex composition of seawater, it is particularly difficult to selectively and efficiently adsorb uranium due to the severe interference of vanadium ions. Therefore, it is crucial to design and synthesize materials for efficient adsorption of uranium from seawater.