https://www.cas.cn/syky/202406/t20240606_5019174.shtml
https://www.nature.com/articles/s41467-024-49095-2
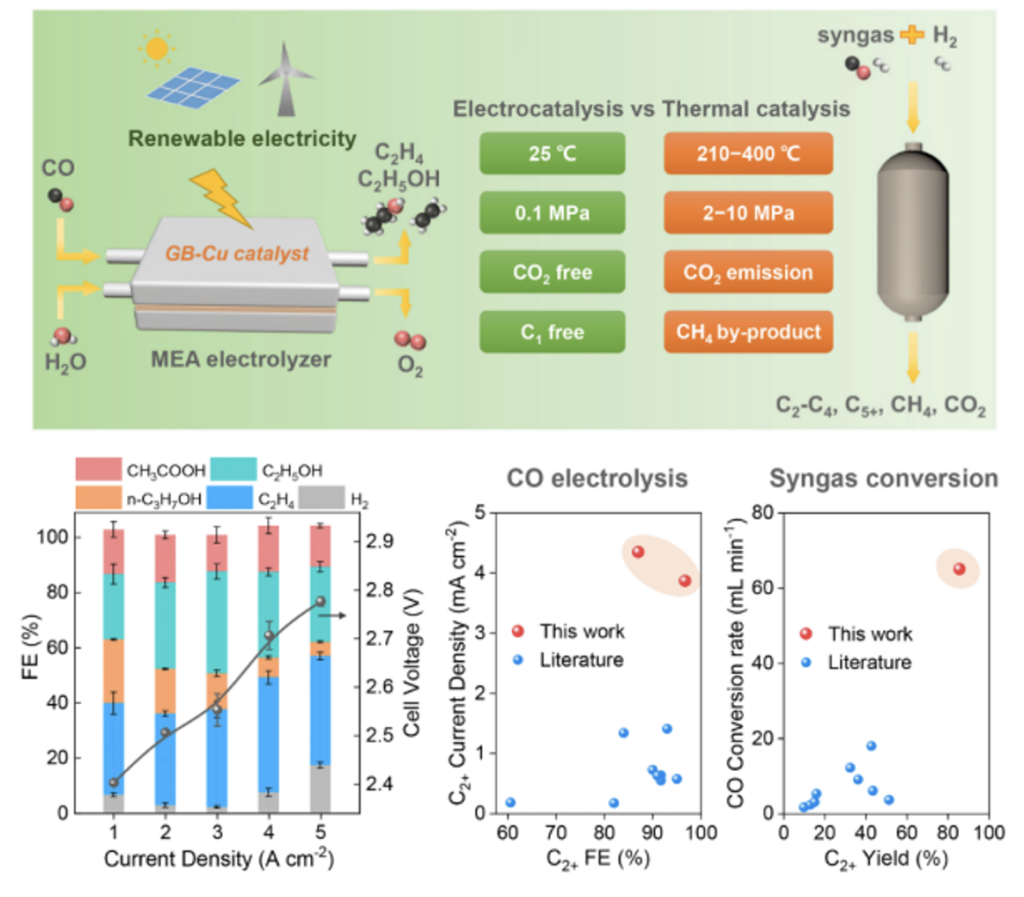
The synthesis of high-value fuels and chemicals such as ethylene using CO derived from coal, natural gas and biomass is an important non-petroleum route. Traditional CO thermal catalytic conversion routes such as Fischer-Tropsch synthesis require the water gas shift reaction to increase the H2/CO ratio, but emit a large amount of CO2. In addition, the synthesis gas conversion reaction usually converts 20% to 50% of CO into CO2 and methane, which increases carbon emissions.
Researchers at the CAS Dalian Institute of Chemical Physics (DICP) have developed an electrocatalytic process CO electrolysis conversion under mild conditions, which uses water as a hydrogen source. It prevents the side reaction path of CO molecules to CO2 by using the reduction electrode potential conditions. The team used a copper catalyst with high-density grain boundaries and an alkaline membrane electrode electrolyzer/stack to achieve efficient CO electrolysis to produce C2+ products. At a total current density of 5A/cm2, the Faraday efficiency of C2+ products reached 87% and no C1 products such as CO2 and methane were generated, and the yield of C2+ products reached 85%. The process has high electrolysis performance and has a higher CO conversion rate and C2+ yield compared with thermal catalytic synthesis gas conversion. The results of working condition Raman spectroscopy and density functional theory calculations show that the abundant grain boundary sites on the copper nanoparticle catalyst promote carbon-carbon coupling. Furthermore, the team assembled 5 sections of 100cm2 alkaline membrane stacks, with a maximum electrolysis power of 5.8kW. At a total current of 400A, the C2+ generation rate was 118.9mmol/min and the ethylene generation rate reached 1.2L/min. This study shows that CO electrolysis is a practical route for the catalytic conversion of CO to produce high-value C2+ fuels and chemicals.