https://www.cas.cn/syky/202406/t20240603_5016508.shtml
https://pubs.acs.org/doi/10.1021/jacs.4c02835
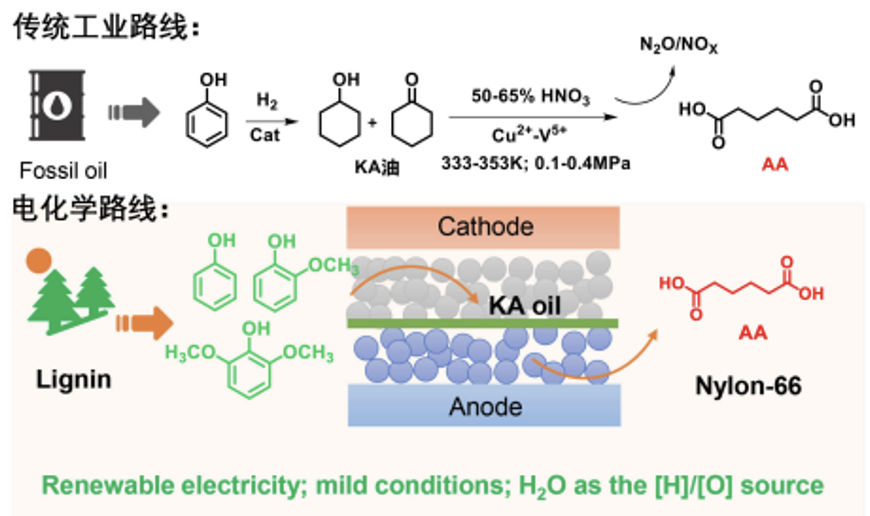
Adipic acid is a basic chemical raw material for various nylon polymers, especially as a monomer for the preparation of nylon-66 and polyurethane. Commercial adipic acid products are all prepared from benzene by thermal catalytic hydrogenation and nitric acid oxidation in two steps.
Chen Yong’s team at the Photochemical Conversion and Synthesis Center of the CAS Institute of Physical and Chemical Technology has used lignin-derived phenolic compounds as raw materials and water as the only hydrogen/oxygen source, using an electrochemical paired electrolysis strategy to achieve green electrosynthesis of adipic acid. The study showed that using PtAu alloy as the cathode catalyst, phenol was efficiently converted into KA oil (a mixture of cyclohexanol and cyclohexanone) with a yield of 92% and a Faraday efficiency of 43%. KA oil was electro-oxidized to adipic acid on the CuCo2O4 anode catalyst with a yield of 85% and a Faraday efficiency of 84%.
KA oil, especially cyclohexanone, has poor solubility in aqueous solution and slow diffusion, making it difficult to enrich and adsorb on the catalyst surface for electrocatalytic oxidation reactions, resulting in poor catalytic activity. In addition, due to mass transfer limitations, the oxygen evolution reaction as a competing reaction will dominate at high current density, thereby reducing the Faraday efficiency and increasing the reaction energy consumption. In response to the above problems, the team proposed a catalyst interface microenvironment regulation strategy and designed and synthesized a cobalt tetraoxide/graphene (Co3O4/GDY) composite electrocatalyst. Taking advantage of the intrinsic all-carbon structure hydrophobicity and abundant acetylenic bonds of GDY, GDY can be used as a catalyst hydrophobicity and active center electronic structure regulator. The results of electrochemical kinetics experiments combined with molecular dynamics simulations show that the composite catalyst can more efficiently enrich and adsorb organic reaction substrates, and improve the reaction rate and Faraday efficiency of KA oil electrooxidation to produce adipic acid. Compared with Co3O4 without GDY modification, the reaction rate of Co3O4/GDY to generate adipic acid increased by 1.6 times, and the Faraday efficiency increased from 73% to 92%.