https://www.cas.cn/syky/202401/t20240124_5001454.shtml
https://doi.org/10.1038/s41557-023-01395-8
Hydrogenation of nitrogen to synthesize ammonia is one of the key chemical reactions for sustaining life on earth and meeting the energy and chemical needs of human society. However, the existing Haber-Bosch technology for ammonia synthesis requires demanding reaction conditions at high temperature and pressure (>400ºC, >100 bar). This is an energy-intensive and carbon-emitting process. The development of new technologies for ammonia synthesis driven by renewable energy sources and implemented under mild conditions is a long-standing goal of researchers and a challenging research topic in the chemical sciences. Solar energy is an inexhaustible renewable energy source, and the realization of light-fed ammonia synthesis process is one of the most ideal ways to synthesize ammonia.
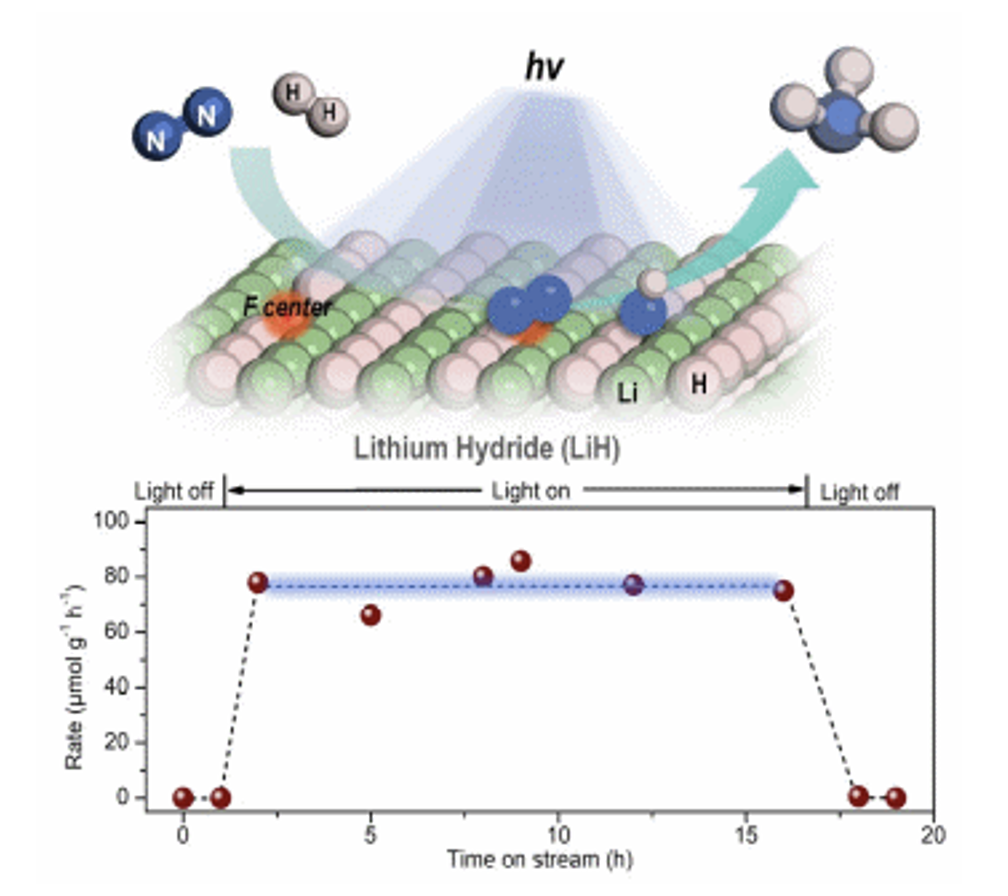
A team at the CAS Dalian Institute of Chemical Physics now realized a lithium hydride-mediated photochemical synthesis of ammonia. The team explored the nitrogen fixation behavior of hydrides under light . It was found that LiH, as an inorganic broad-band semiconductor, dehydrogenates and changes color under UV illumination. Unlike conventional oxide or nitride semiconductors, after LiH generates carrier separation, negative hydrogen (H‾) loses electrons to form H2 and generates hydrogen vacancies, and photogenerated electrons can form electron-rich color-centered structures in the surface hydrogen vacancies, which contribute to nitrogen reduction activation. In this process, negative hydrogen can participate in the formation of N-H bonds. Under nitrogen-hydrogen co-feeding conditions, the team realized the LiH photocatalytic ammonia synthesis process under mild conditions. This work demonstrates the development potential of hydrides in mediating photochemical reactions and enriches the body of knowledge of hydride nitrogen fixation chemistry.